JWH-164
外观
 | |
| 法律規範狀態 | |
|---|---|
| 法律規範 |
|
| 识别信息 | |
| |
| CAS号 | 824961-61-7 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C25H25NO2 |
| 摩尔质量 | 371.48 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
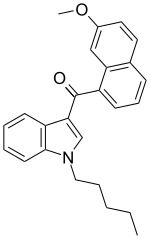
JWH-164,化学名N-正戊基-3-(7-甲氧基-1-萘甲酰基)吲哚,分子式C
25H
25NO
2,属于萘甲酰基吲哚类JWH系列大麻素,是大麻素受体CB1和CB2的激动剂[1][2]。它可以7-甲氧基-1-萘甲酸和N-戊基吲哚为原料经多步反应制得。[3]
参考文献
[编辑]- ^ Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorganic & Medicinal Chemistry. January 2005, 13 (1): 89–112. PMID 15582455. doi:10.1016/j.bmc.2004.09.050.
- ^ Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Current Medicinal Chemistry. 2005, 12 (12): 1395–411. PMID 15974991. doi:10.2174/0929867054020864.
- ^ Huffman, John W.; et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB1 and CB2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB2 receptor agonists. Bioorganic & Medicinal Chemistry (2004), 13 (1), 89-112. doi:10.1016/j.bmc.2004.09.050.
