User:Njzjz/沙盒2
| 這是Njzjz的用户页。用户沙盒是用户页的子頁面,属于用户的測試區,不是維基百科條目。 公用沙盒:主沙盒 | 使用指南沙盒一、二 | 模板沙盒 | 更多…… 此用户页的子頁面: 外觀選項: 用字選項: 如果您已经完成草稿,可以请求志愿者协助将其移动到条目空间。 |
Template:Nanomat Template:Nanotech
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 1000 nanometres (10−9 meter) but is usually 1—100 nm (the usual definition of nanoscale[1]).
Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, or mechanical properties.[2]
Nanomaterials are slowly becoming commercialized[3] and beginning to emerge as commodities.[4]
Types
[编辑]Natural nanomaterials
[编辑]Biological systems often feature natural, functional nanomaterials. The structure of foraminifera (mainly chalk) and viruses (protein, capsid), the wax crystals covering a lotus or nasturtium leaf, spider and spider-mite silk,[5] the blue hue of tarantulas,[6] the "spatulae" on the bottom of gecko feet, some butterfly wing scales, natural colloids (milk, blood), horny materials (skin, claws, beaks, feathers, horns, hair), paper, cotton, nacre, corals, and even our own bone matrix are all natural organic nanomaterials.
Natural inorganic nanomaterials occur through crystal growth in the diverse chemical conditions of the Earth's crust. For example, clays display complex nanostructures due to anisotropy of their underlying crystal structure, and volcanic activity can give rise to opals, which are an instance of a naturally occurring photonic crystals due to their nanoscale structure. Fires represent particularly complex reactions and can produce pigments, cement, fumed silica etc.
-
Viral capsid
-
"Lotus effect", hydrophobic effect with self-cleaning ability
-
Close-up of the underside of a gecko's foot as it walks on a glass wall. (spatula: 200 × 10-15 nm).
-
SEM micrograph of a butterfly wing scale (× 5000)
-
Peacock feather (detail)
-
Brazilian Crystal Opal. The play of color is caused by the interference and diffraction of light between silica spheres (150 - 300 nm in diameter).
-
Lycurgus Cup, glass, 4th century, Roman. Nanoparticles (70 nm) of gold and silver, dispersed in colloidal form, are responsible for the dichroic effect (red/green).
-
Blue hue of a species of tarantula (450 nm ± 20 nm)
Fullerenes
[编辑]The fullerenes are a class of allotropes of carbon which conceptually are graphene sheets rolled into tubes or spheres. These include the carbon nanotubes (or silicon nanotubes) which are of interest both because of their mechanical strength and also because of their electrical properties.[7]
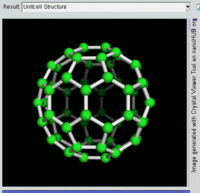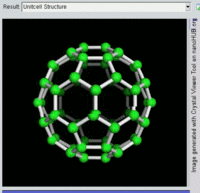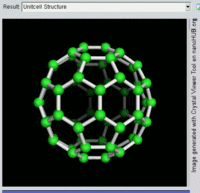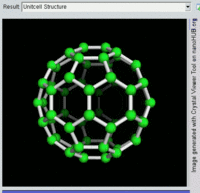
The first fullerene molecule to be discovered, and the family's namesake, buckminsterfullerene (C60), was prepared in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University. The name was a homage to Buckminster Fuller, whose geodesic domes it resembles. Fullerenes have since been found to occur in nature.[8] More recently, fullerenes have been detected in outer space.[9]
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure of resistant bacteria and even target certain types of cancer cells such as melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated antimicrobial agents. In the field of nanotechnology, heat resistance and superconductivity are among the properties attracting intense research.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By DFT and TDDFT methods one can obtain IR, Raman and UV spectra. Results of such calculations can be compared with experimental results.
Graphene nanostructures
[编辑]2D materials are crystalline materials consisting of a two-dimensional single layer of atoms. The most important representative graphene was discovered in 2004.
Box-shaped graphene (BSG) nanostructure is an example of 3D nanomaterial.[10] BSG nanostructure has appeared after mechanical cleavage of pyrolytic graphite. This nanostructure is a multilayer system of parallel hollow nanochannels located along the surface and having quadrangular cross-section. The thickness of the channel walls is approximately equal to 1 nm. The typical width of channel facets makes about 25 nm.
Nanoparticles
[编辑]Inorganic nanomaterials, (e.g. quantum dots, nanowires and nanorods) because of their interesting optical and electrical properties, could be used in optoelectronics.[11] Furthermore, the optical and electronic properties of nanomaterials which depend on their size and shape can be tuned via synthetic techniques. There are the possibilities to use those materials in organic material based optoelectronic devices such as Organic solar cells, OLEDs etc. The operating principles of such devices are governed by photoinduced processes like electron transfer and energy transfer. The performance of the devices depends on the efficiency of the photoinduced process responsible for their functioning. Therefore, better understanding of those photoinduced processes in organic/inorganic nanomaterial composite systems is necessary in order to use them in organic optoelectronic devices.
Nanoparticles or nanocrystals made of metals, semiconductors, or oxides are of particular interest for their mechanical, electrical, magnetic, optical, chemical and other properties.[12][13] Nanoparticles have been used as quantum dots and as chemical catalysts such as nanomaterial-based catalysts. Recently, a range of nanoparticles are extensively investigated for biomedical applications including tissue engineering, drug delivery, biosensor.[14]
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecular structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as quantum confinement in semiconductor particles, surface plasmon resonance in some metal particles and superparamagnetism in magnetic materials.
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same malleability and ductility as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. Suspensions of nanoparticles are possible because the interaction of the particle surface with the solvent is strong enough to overcome differences in density, which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visual properties because they are small enough to confine their electrons and produce quantum effects. For example, gold nanoparticles appear deep red to black in solution.
The often very high surface area to volume ratio of nanoparticles provides a tremendous driving force for diffusion, especially at elevated temperatures. Sintering is possible at lower temperatures and over shorter durations than for larger particles. This theoretically does not affect the density of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate do complicate matters. The surface effects of nanoparticles also reduces the incipient melting temperature.
Nanozymes
[编辑]Nanozymes are nanomaterials with enzyme-like characteristics.[15] They are an emerging type of artificial enzyme, which have been used for wide applications in such as biosensing, bioimaging, tumor diagnosis and therapy, antibiofouling, etc.
Synthesis
[编辑]The goal of any synthetic method for nanomaterials is to yield a material that exhibits properties that are a result of their characteristic length scale being in the nanometer range (~1 – 100 nm). Accordingly, the synthetic method should exhibit control of size in this range so that one property or another can be attained. Often the methods are divided into two main types "Bottom Up" and "Top Down."
Bottom up methods
[编辑]Bottom up methods involve the assembly of atoms or molecules into nanostructured arrays. In these methods the raw material sources can be in the form of gases, liquids or solids. The latter requiring some sort of disassembly prior to their incorporation onto a nanostructure. Bottom methods generally fall into two categories: chaotic and controlled.
Chaotic processes
[编辑]Chaotic processes involve elevating the constituent atoms or molecules to a chaotic state and then suddenly changing the conditions so as to make that state unstable. Through the clever manipulation of any number of parameters, products form largely as a result of the insuring kinetics. The collapse from the chaotic state can be difficult or impossible to control and so ensemble statistics often govern the resulting size distribution and average size. Accordingly, nanoparticle formation is controlled through manipulation of the end state of the products.
Examples of Chaotic Processes are: Laser ablation, Exploding wire, Arc, Flame pyrolysis, Combustion, Precipitation synthesis techniques.
Controlled processes
[编辑]Controlled Processes involve the controlled delivery of the constituent atoms or molecules to the site(s) of nanoparticle formation such that the nanoparticle can grow to a prescribed sizes in a controlled manner. Generally the state of the constituent atoms or molecules are never far from that needed for nanoparticle formation. Accordingly, nanoparticle formation is controlled through the control of the state of the reactants.
Examples of controlled processes are self-limiting growth solution, self-limited chemical vapor deposition, shaped pulse femtosecond laser techniques, and molecular beam epitaxy.
Characterization
[编辑]Novel effects can occur in materials when structures are formed with sizes comparable to any one of many possible length scales, such as the de Broglie wavelength of electrons, or the optical wavelengths of high energy photons. In these cases quantum mechanical effects can dominate material properties. One example is quantum confinement where the electronic properties of solids are altered with great reductions in particle size. The optical properties of nanoparticles, e.g. fluorescence, also become a function of the particle diameter. This effect does not come into play by going from macrosocopic to micrometer dimensions, but becomes pronounced when the nanometer scale is reached.
In addition to optical and electronic properties, the novel mechanical properties of many nanomaterials is the subject of nanomechanics research. When added to a bulk material, nanoparticles can strongly influence the mechanical properties of the material, such as the stiffness or elasticity. For example, traditional polymers can be reinforced by nanoparticles (such as carbon nanotubes) resulting in novel materials which can be used as lightweight replacements for metals. Such composite materials may enable a weight reduction accompanied by an increase in stability and improved functionality.[16]
Finally, nanostructured materials with small particle size such as zeolites, and asbestos, are used as catalysts in a wide range of critical industrial chemical reactions. The further development of such catalysts can form the basis of more efficient, environmentally friendly chemical processes.
The first observations and size measurements of nano-particles were made during the first decade of the 20th century. Zsigmondy made detailed studies of gold sols and other nanomaterials with sizes down to 10 nm and less. He published a book in 1914.[17] He used an ultramicroscope that employs a dark field method for seeing particles with sizes much less than light wavelength.
There are traditional techniques developed during 20th century in Interface and Colloid Science for characterizing nanomaterials. These are widely used for first generation passive nanomaterials specified in the next section.
These methods include several different techniques for characterizing particle size distribution. This characterization is imperative because many materials that are expected to be nano-sized are actually aggregated in solutions. Some of methods are based on light scattering. Others apply ultrasound, such as ultrasound attenuation spectroscopy for testing concentrated nano-dispersions and microemulsions.[18]
There is also a group of traditional techniques for characterizing surface charge or zeta potential of nano-particles in solutions. This information is required for proper system stabilzation, preventing its aggregation or flocculation. These methods include microelectrophoresis, electrophoretic light scattering and electroacoustics. The last one, for instance colloid vibration current method is suitable for characterizing concentrated systems.
Uniformity
[编辑]The chemical processing and synthesis of high performance technological components for the private, industrial and military sectors requires the use of high purity ceramics, polymers, glass-ceramics and material composites. In condensed bodies formed from fine powders, the irregular sizes and shapes of nanoparticles in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact.
Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to in microstructural inhomogeneities. Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to crack propagation in the unfired body if not relieved.[19][20] [21]
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the sintering process, yielding inhomogeneous densification. Some pores and other structural defects associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws. [22][23]
It would therefore appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions which will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over particle-particle interactions. It should be noted here that a number of dispersants such as ammonium citrate (aqueous) and imidazoline or oleyl alcohol (nonaqueous) are promising solutions as possible additives for enhanced dispersion and deagglomeration. Monodisperse nanoparticles and colloids provide this potential.[24]
Monodisperse powders of colloidal silica, for example, may therefore be stabilized sufficiently to ensure a high degree of order in the colloidal crystal or polycrystalline colloidal solid which results from aggregation. The degree of order appears to be limited by the time and space allowed for longer-range correlations to be established. Such defective polycrystalline colloidal structures would appear to be the basic elements of sub-micrometer colloidal materials science, and, therefore, provide the first step in developing a more rigorous understanding of the mechanisms involved in microstructural evolution in high performance materials and components. [25][26]
Legal definition
[编辑]On 18 October 2011, the European Commission adopted the following definition of a nanomaterial: "A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm – 100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1 and 50%."[27]
However, this differs from the definition adopted by the International Organization for Standardization (ISO), which is: "Material with any external dimension in the nanoscale or having internal structure in the nanoscale."[28]
"Nanoscale" is, in turn, defined as: "Size range from approximately 1 nm to 100 nm."[28]
It is not currently known which of these, if any, will prevail in courts of law.
Safety
[编辑]Health impact
[编辑]While nanomaterials and nanotechnologies are expected to yield numerous health and health care advances, such as more targeted methods of delivering drugs, new cancer therapies, and methods of early detection of diseases, they also may have unwanted effects.[29][30] Increased rate of absorption is the main concern associated with manufactured nanoparticles.
When materials are made into nanoparticles, their surface area to volume ratio increases. The greater specific surface area (surface area per unit weight) may lead to increased rate of absorption through the skin, lungs, or digestive tract and may cause unwanted effects to the lungs as well as other organs. However, the particles must be absorbed in sufficient quantities in order to pose health risks.[30]
Nanoparticles created adventitiously (e.g., through the rubbing of prostheses) have long been known to be a health hazard,[31] but as the use of nanomaterials increases worldwide, concerns for worker and user safety are mounting. To address such concerns, the Swedish Karolinska Institute conducted a study in which various nanoparticles were introduced to human lung epithelial cells. The results, released in 2008, showed that iron oxide nanoparticles caused little DNA damage and were non-toxic. Zinc oxide nanoparticles were slightly worse. Titanium dioxide caused only DNA damage. Carbon nanotubes caused DNA damage at low levels. Copper oxide was found to be the worst offender, and was the only nanomaterial identified by the researchers as a clear health risk.[32] Though nanomaterials are not confirmed as a health risk to workers who produce them, NIOSH recommends that exposure precautions and personal protective equipment be used to protect workers until the risks of nanomaterial manufacture are better understood.[33][34]
Occupational safety
[编辑]Beyond normal chemical safety practices such as using personal protective equipment, The United States National Institute for Occupational Safety and Health recommends the use of HEPA filtration on local exhaust ventilation such as fume hoods. General laboratory ventilation is often not sufficient to effectively clear nanomaterials released into the general room air over a 30-minute period; therefore, researchers should leave the hood fan on even after synthesis is complete. Nanomaterials in dry powder form have the most risk of airborne dust generation and dermal contact, followed by liquid suspensions and then by nanomaterials embedded in a solid matrix.[35]
Nanoparticles behave differently than other similarly sized particles. It is therefore necessary to develop specialized approaches to testing and monitoring their effects on human health and on the environment. The OECD Chemicals Committee has established the Working Party on Manufactured Nanomaterials to address this issue and to study the practices of OECD member countries in regards to nanomaterial safety.[36]
See also
[编辑]References
[编辑]- ^ Buzea, Cristina; Pacheco, Ivan; Robbie, Kevin. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases. 2007, 2 (4): MR17–MR71. PMID 20419892. doi:10.1116/1.2815690.
- ^ Hubler, A.; Osuagwu, O. Digital quantum batteries: Energy and information storage in nanovacuum tube arrays. Complexity. 2010. doi:10.1002/cplx.20306.
- ^ Eldridge, T. Achieving industry integration with nanomaterials through financial markets. Nanotechnology_Now. 8 January 2014.
- ^ McGovern, C. Commoditization of nanomaterials. Nanotechnol. Perceptions. 2010, 6 (3): 155–178. doi:10.4024/N15GO10A.ntp.06.03.
- ^ Novel natural nanomaterial spins off from spider-mite genome sequencing. Phys.Org (May 23, 2013)
- ^ Why Are Tarantulas Blue?.
- ^ Fullerenes. Encyclopædia Britannica.
- ^ Buseck, P.R.; Tsipursky, S.J.; Hettich, R. Fullerenes from the Geological Environment. Science. 1992, 257 (5067): 215–7. Bibcode:1992Sci...257..215B. PMID 17794751. doi:10.1126/science.257.5067.215.
- ^ Cami, J; Bernard-Salas, J.; Peeters, E.; Malek, S. E. Detection of C60 and C70 in a Young Planetary Nebula. Science. 2 September 2010, 329 (5996): 1180–2. Bibcode:2010Sci...329.1180C. PMID 20651118. doi:10.1126/science.1192035.
- ^ R. V. Lapshin. STM observation of a box-shaped graphene nanostructure appeared after mechanical cleavage of pyrolytic graphite (PDF). Applied Surface Science (Netherlands: Elsevier B. V.). 2016, 360: 451–460. ISSN 0169-4332. doi:10.1016/j.apsusc.2015.09.222.
- ^ Zeng, S.; Baillargeat, Dominique; Ho, Ho-Pui; Yong, Ken-Tye. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chemical Society Reviews. 2014, 43 (10): 3426–3452. PMID 24549396. doi:10.1039/C3CS60479A.
- ^ Stephenson, C.; Hubler, A. Stability and conductivity of self assembled wires in a transverse electric field. Sci.Rep.5. 2015. doi:10.1038/srep15044.
- ^ Hubler, A.; Lyon, D. Gap size dependence of the dielectric strength in nano vacuum gaps. IEEE. 2013. doi:10.1109/TDEI.2013.6571470.
- ^ Kerativitayanan, P; Carrow, JK; Gaharwar, AK. Nanomaterials for Engineering Stem Cell Responses.. Advanced healthcare materials. 26 May 2015, 4: 1600–27. PMID 26010739. doi:10.1002/adhm.201500272.
- ^ Wei, Hui; Wang, Erkang. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews. 2013-06-21, 42 (14): 6060. ISSN 1460-4744. doi:10.1039/C3CS35486E.
- ^ Ramsden, J.J. (2011) Nanotechnology: An Introduction, Elsevier, Amsterdam
- ^ Zsigmondy, R. (1914) "Colloids and the Ultramicroscope", J. Wiley and Sons, NY
- ^ Dukhin, A.S. & Goetz, P.J. Ultrasound for characterizing colloids. Elsevier. 2002.
- ^ Onoda, G.Y. Jr.; Hench, L.L. (编). Ceramic Processing Before Firing. New York: Wiley & Sons. 1979. ISBN 0-471-65410-8.
- ^ Aksay, I.A.; Lange, F.F.; Davis, B.I. Uniformity of Al2O3-ZrO2 Composites by Colloidal Filtration. J. Am. Ceram. Soc. 1983, 66 (10): C–190. doi:10.1111/j.1151-2916.1983.tb10550.x.
- ^ Franks, G.V. & Lange, F.F. Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts. J. Am. Ceram. Soc. 1996, 79 (12): 3161–3168. doi:10.1111/j.1151-2916.1996.tb08091.x.
- ^ Evans, A.G.; Davidge, R.W. The strength and fracture of fully dense polycrystalline magnesium oxide. Phil. Mag. 1969, 20 (164): 373–388. Bibcode:1969PMag...20..373E. doi:10.1080/14786436908228708.
- ^ Lange, F.F. & Metcalf, M. Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering. J. Am. Ceram. Soc. 1983, 66 (6): 398–406. doi:10.1111/j.1151-2916.1983.tb10069.x.
- ^ Evans, A.G. Considerations of Inhomogeneity Effects in Sintering. J. Am. Ceram. Soc. 1987, 65 (10): 497–501. doi:10.1111/j.1151-2916.1982.tb10340.x.
- ^ Whitesides, George M.; et al. Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures. Science. 1991, 254 (5036): 1312–9. Bibcode:1991Sci...254.1312W. PMID 1962191. doi:10.1126/science.1962191.
- ^ Dubbs D. M; Aksay I.A. Self-Assembled Ceramics Produced by Complex-Fluid Templation. Annu. Rev. Phys. Chem. 2000, 51: 601–22. Bibcode:2000ARPC...51..601D. PMID 11031294. doi:10.1146/annurev.physchem.51.1.601.
- ^ Nanomaterials. European Commission. Last updated 18 October 2011
- ^ 28.0 28.1 ISO/TS 800004-1 Nanotechnologies—Vocabulary—Part 1: Core terms. Geneva: 2011.
- ^ Kerativitayanan, Punyavee; Carrow, James K.; Gaharwar, Akhilesh K. Nanomaterials for Engineering Stem Cell Responses. Advanced Healthcare Materials. May 2015, 4: 1600–27. PMID 26010739. doi:10.1002/adhm.201500272.
- ^ 30.0 30.1 Lauterwasser, C. Opportunities and risks of Nanotechnologies (PDF). OECD.org. 18 July 2007.
- ^ Revell, P.A. The biological effects of nanoparticles. Nanotechnology Perceptions. 2006, 2 (3): 283–298. ISSN 1660-6795.
- ^ Study Sizes up Nanomaterial Toxicity. Chemical & Engineering News. 2008, 86 (35): 44.
- ^ Topmiller, Jennifer L.; Dunn, Kevin H. Controlling Exposures to Workers Who Make or Use Nanomaterials. National Institute of Occupational Safety and Health. 9 December 2013 [6 January 2015].
- ^ Current Strategies for Engineering Controls in Nanomaterial Production and Downstream Handling Processes (PDF). National Institute of Occupational Safety and Health. November 2013 [6 January 2015].
- ^ General Safe Practices for Working with Engineered Nanomaterials in Research Laboratories. National Institute of Occupational Safety and Health: 6–8. May 2012 [2016-07-15].
- ^ Safety of Manufactured Nanomaterials: About, OECD Environment Directorate. OECD.org. 18 July 2007.
External links
[编辑]- Acquisition, evaluation and public orientated presentation of societal relevant data and findings for nanomaterials (DaNa)
- Safety of Manufactured Nanomaterials: OECD Environment Directorate
- Assessing health risks of nanomaterials summary by GreenFacts of the European Commission SCENIHR assessment
- International Liposome Society
- Textiles Nanotechnology Laboratory at Cornell University
- IOP.org Article
- Nano Structured Material
- Online course MSE 376-Nanomaterials by Mark C. Hersam (2006)
- Nanomaterials: Quantum Dots, Nanowires and Nanotubes online presentation by Dr Sands
- Lecture Videos for the Second International Symposium on the Risk Assessment of Manufactured Nanomaterials, NEDO 2012
- Nader Engheta: Wave interaction with metamaterials, SPIE Newsroom 2016








