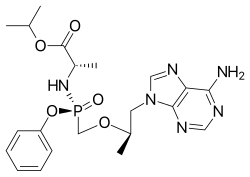
丙酚替诺福韦
外观
 | |
| 临床资料 | |
|---|---|
| 读音 | /ˌtəˈnoʊfəvɪər ˌæləˈfɛnəmaɪd/ |
| 商品名 | 韦立德(Vemlidy) |
| 其他名称 | GS-7340 |
| AHFS/Drugs.com | Monograph |
| 核准状况 |
|
| 怀孕分级 | |
| 给药途径 | 口服 |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 血浆蛋白结合率 | ~80%[3] |
| 生物半衰期 | 33分钟 |
| 排泄途径 | 肠道排泄(31.7%)和肾脏排泄(<1%) |
| 识别信息 | |
| |
| CAS号 | 379270-37-8 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C21H29N6O5P |
| 摩尔质量 | 476.47 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
丙酚替诺福韦(Tenofovir alafenamide,缩写TAF,商品名韦立德(Vemlidy))是一种口服抗逆转录病毒药物,临床上应用其延胡索酸盐的形式(富马酸丙酚替诺福韦) 用于治疗乙型肝炎或与其他药物联用治疗艾滋病。
该药为替诺福韦的前药,由吉利德科学研发。与常用的逆转录酶抑制剂替诺福韦二吡呋酯(TDF)相比,丙酚替诺福韦具有更强的抗病毒活性和更好的淋巴组织分布。[5][6]该药于2016年11月获得FDA批准上市。[7]
相关复方药物
[编辑]- 恩曲他滨/丙酚替诺福韦 [8] — 2016 年 4 月在美国获得批准治疗HIV。2019年10月,该药在美国被批准用于HIV-1暴露前预防(PrEP)。 [9] [10]
- 达芦那韦/考比司他/恩曲他滨/丙酚替诺福韦[11] — 欧盟于2017年9月、美国于2018年7月、澳大利亚于2019年11月相继批准该药上市。[12][13][14]
- 多替拉韦/恩曲他滨/丙酚替诺福韦。[15]
参考文献
[编辑]- ^ 1.0 1.1 Tenofovir alafenamide (Vemlidy) Use During Pregnancy. Drugs.com. 26 December 2018 [18 April 2020]. (原始内容存档于2021-07-09).
- ^ Vemlidy 25 mg film coated tablets - Summary of Product Characteristics (SmPC). (emc). 8 September 2020 [12 November 2020]. (原始内容存档于2021-07-11).
- ^ 3.0 3.1 Vemlidy- tenofovir alafenamide tablet. DailyMed. 11 February 2020 [18 April 2020]. (原始内容存档于2021-07-09).
- ^ Vemlidy EPAR. European Medicines Agency (EMA). [2021-07-01]. (原始内容存档于2021-07-13).
- ^ Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids. 2001, 20 (4–7): 1091–8. PMID 11562963. doi:10.1081/NCN-100002496.
- ^ M Markowitz, A Zolopa, et al.
- ^ FDA Approves Vemlidy (tenofovir alafenamide) for Chronic Hepatitis B in Adults. United States Department of Health and Human Services. 21 November 2016 [11 October 2019]. (原始内容存档于11 October 2019).
- ^ Descovy- emtricitabine and tenofovir alafenamide tablet. DailyMed. 13 January 2020 [18 April 2020]. (原始内容存档于2021-06-24).
- ^ FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic. U.S. Food and Drug Administration (FDA). 3 October 2019 [11 October 2019]. (原始内容存档于11 October 2019).
- ^ Mandavilli, Apoorva. F.D.A. Approves New H.I.V.-Prevention Drug, but Not for Everyone. The New York Times. 4 October 2019 [11 October 2019]. (原始内容存档于2021-07-09).
- ^ Symtuza- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coated. DailyMed. 6 March 2020 [18 April 2020]. (原始内容存档于2021-07-09).
- ^ Drug Approval Package: Symtuza (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide). U.S. Food and Drug Administration (FDA). 11 December 2018 [19 August 2020]. (原始内容存档于2021-07-09).
- ^ Symtuza EPAR. European Medicines Agency. [19 August 2020]. (原始内容存档于2021-07-10).
- ^ http://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=CCE11C6BC5177A30CA2585AE00423857&agid=(PrintDetailsPublic)&actionid=1[失效链接]
- ^ Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration (FDA). [5 December 2020]. (原始内容存档于2021-07-09).
外部链接
[编辑]- Tenofovir alafenamide. Drug Information Portal. U.S. National Library of Medicine. [2021-07-01]. (原始内容存档于2021-06-24).
- Tenofovir alafenamide fumarate. Drug Information Portal. U.S. National Library of Medicine. [2021-07-01]. (原始内容存档于2021-07-09).
