用戶:A22234798/History of chemistry
Template:History of science sidebar
By 1000 BC, the ancient civilizations were using technologies that would form the basis of the various branches of chemistry. Extracting metal from their ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from plants for medicine and perfume, making cheese, dying cloth, tanning leather, rendering fat into soap, making glass, and making alloys like bronze.
Philosophical attempts to explain the nature of matter and its transformations failed. The protoscience of alchemy also failed, but by experimentation and recording the results set the stage for science. Modern chemistry begins to emerge when a clear distinction is made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). Chemistry then becomes a full-fledged science when Antoine Lavoisier develops his law of conservation of mass, which demands careful measurements and quantitative observations of chemical phenomena. So, while both alchemy and chemistry are concerned with the nature of matter and its transformations, it is only the chemists who apply the scientific method. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.[1]
From fire to atomism
[編輯]Arguably the first chemical reaction that was used in a controlled manner by mankind was fire. However, for millennia, in absence of a scientific understanding, fire was simply a mystical force that could transform one substance into another (burn the wood, or boil the water) while producing heat and light. Fire affected many aspects of early societies, ranging from the most simple facets of everyday life, such as cooking and habitat lighting, to more advanced technologies, such as pottery, bricks, and smelting of metals to make tools.
Philosophical attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry, can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary elements that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as ideas, aether, and heaven, were common in ancient civilizations even in absence of any cross-fertilization; for example in Greek, Indian, Mayan, and ancient Chinese philosophies all considered air, water, earth and fire as primary elements.[來源請求]
Atomism can be traced back to ancient Greece and ancient India.[2] Greek atomism dates back to 440 BC, as what might be indicated by the book De Rerum Natura (The Nature of Things)[3] written by the Roman Lucretius[4] in 50 BC. In the book was found ideas traced back to Democritus and Leucippus, who declared that atoms were the most indivisible part of matter. This coincided with a similar declaration by Indian philosopher Kanada in his Vaisheshika sutras around the same time period.[2] By similar means discussed the existence of gases. What Kanada declared by sutra, Democritus declared by philosophical musing. Both suffered from a lack of empirical data. Without scientific proof, the existence of atoms was easy to deny. Aristotle opposed the existence of atoms in 330 BC; and the atomism of the Vaisheshika school was also opposed for a long time.[來源請求]
Much of the early development of purification methods is described by Pliny the Elder in his Naturalis Historia. He made attempts to explain those methods, as well as making acute observations of the state of many minerals.
The rise of metallurgy
[編輯]It was fire that led to the discovery of glass and the purification of metals which in turn gave way to the rise of metallurgy.[來源請求] During the early stages of metallurgy, methods of purification of metals were sought, and gold, known in ancient Egypt as early as 2600 BC, became a precious metal. The discovery of alloys heralded the Bronze Age. After the Bronze Age, the history of metallurgy was marked by which army had better weaponry. Countries in Eurasia had their heyday when they made the superior alloys, which, in turn, made better armour and better weapons. This often determined the outcomes of battles.[來源請求] Significant progress in metallurgy and alchemy was made in ancient India.[5]
The philosopher's stone and the rise of alchemy
[編輯]
Many people were interested in finding a method that could convert cheaper metals into gold. The material that would help them do this was rumored to exist in what was called the philosopher's stone. This led to the protoscience called alchemy. Alchemy was practiced by many cultures throughout history and often contained a mixture of philosophy, mysticism, and protoscience.[來源請求]
Alchemy not only sought to turn base metals into gold, but especially in a Europe rocked by bubonic plague, there was hope that alchemy would lead to the development of medicines to improve people's health. The holy grail of this strain of alchemy was in the attempts made at finding the elixir of life, which promised eternal youth. Neither the elixir nor the philosopher's stone were ever found. Also, characteristic of alchemists was the belief that there was in the air an "ether" which breathed life into living things.[來源請求] Practitioners of alchemy included Isaac Newton, who remained one throughout his life.
Problems encountered with alchemy
[編輯]There were several problems with alchemy, as seen from today's standpoint. There was no systematic naming system for new compounds, and the language was esoteric and vague to the point that the terminologies meant different things to different people. In fact, according to The Fontana History of Chemistry (Brock, 1992):
The language of alchemy soon developed an arcane and secretive technical vocabulary designed to conceal information from the uninitiated. To a large degree, this language is incomprehensible to us today, though it is apparent that readers of Geoffery Chaucer's Canon's Yeoman's Tale or audiences of Ben Jonson's The Alchemist were able to construe it sufficiently to laugh at it.[6]
Chaucer's tale exposed the more fraudulent side of alchemy, especially the manufacture of counterfeit gold from cheap substances. Soon after Chaucer, Dante Alighieri also demonstrated an awareness of this fraudulence, causing him to consign all alchemists to the Inferno in his writings. Soon after, in 1317, the Avignon Pope John XXII ordered all alchemists to leave France for making counterfeit money. A law was passed in England in 1403 which made the "multiplication of metals" punishable by death. Despite these and other apparently extreme measures, alchemy did not die. Royalty and privileged classes still sought to discover the philosopher's stone and the elixir of life for themselves.[7]
There was also no agreed-upon scientific method for making experiments reproducible. Indeed many alchemists included in their methods irrelevant information such as the timing of the tides or the phases of the moon. The esoteric nature and codified vocabulary of alchemy appeared to be more useful in concealing the fact that they could not be sure of very much at all. As early as the 14th century, cracks seemed to grow in the facade of alchemy; and people became sceptical.[來源請求] Clearly, there needed to be a scientific method where experiments can be repeated by other people, and results needed to be reported in a clear language that laid out both what is known and unknown.
From alchemy to chemistry
[編輯]Early chemists
[編輯]
In the Arab world, the Muslims were translating the works of the ancient Greeks into Arabic and were experimenting with scientific ideas.[8] The development of the modern scientific method was slow and arduous, but an early scientific method for chemistry began emerging among early Muslim chemists, beginning with the 9th century chemist Jabir ibn Hayyan (known as "Geber" in Europe), who is "considered by many to be the father of chemistry".[9][10][11][12] He introduced a systematic and experimental approach to scientific research based in the laboratory, in contrast to the ancient Greek and Egyptian alchemists whose works were often allegorical and unintelligble.[13] He also invented and named the alembic (al-anbiq), chemically analyzed many chemical substances, composed lapidaries, distinguished between alkalis and acids, and manufactured hundreds of drugs.[14]
Among other influential Muslim chemists, Ja'far al-Sadiq,[15] Alkindus,[16] Abū al-Rayhān al-Bīrūnī,[17] Avicenna[18] and Ibn Khaldun refuted the practice of alchemy and the theory of the transmutation of metals; and Tusi described an early version of the conservation of mass, noting that a body of matter is able to change but is not able to disappear.[19] Rhazes refuted Aristotle's theory of four classical elements for the first time and set up the firm foundations of modern chemistry, using the laboratory in the modern sense, designing and describing more than twenty instruments, many parts of which are still in use today, such as a crucible, decensory, cucurbit or retort for distillation, and the head of a still with a delivery tube (ambiq, Latin alembic), and various types of furnace or stove.[20]

For the more honest practitioners in Europe, alchemy became an intellectual pursuit after early Arabic alchemy became available through Latin translation, and over time, they got better at it. Paracelsus (1493-1541), for example, rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called iatrochemistry. Paracelsus was not perfect in making his experiments truly scientific. For example, as an extension of his theory that new compounds could be made by combining mercury with sulfur, he once made what he thought was "oil of sulfur". This was actually dimethyl ether, which had neither mercury nor sulfur.[來源請求]
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists, among them Georg Agricola (1494–1555), who published his great work De re metallica in 1556. His approach removed the mysticism associated with the subject, creating the practical base upon which others could build. The work describes the many kinds of furnace used to smelt ore, and stimulated interest in minerals and their composition. It is no coincidence that he gives numerous references to the earlier author, Pliny the Elder and his Naturalis Historia.
In 1605, Sir Francis Bacon published The Proficience and Advancement of Learning, which contains a description of what would later be known as the scientific method.[21] In 1615 Jean Beguin publishes the Tyrocinium Chymicum, an early chemistry textbook, and in it draws the first-ever chemical equation.[22]

Robert Boyle (1627–1691) is considered to have refined the modern scientific method for alchemy and to have separated chemistry further from alchemy.[23] Robert Boyle was an atomist, but favoured the word corpuscle over atoms. He comments that the finest division of matter where the properties are retained is at the level of corpuscles.
Boyle was credited with the discovery of Boyle's Law. He is also credited for his landmark publication The Sceptical Chymist, where he attempts to develop an atomic theory of matter, with no small degree of success. Despite all these advances, the person celebrated as the "father of modern chemistry" is Antoine Lavoisier who developed his law of conservation of mass in 1789, also called Lavoisier's Law.[24] With this, chemistry acquired a strict quantitative nature, allowing reliable predictions to be made.
In 1754, Joseph Black isolated carbon dioxide, which he called "fixed air".[25] Carl Wilhelm Scheele and Joseph Priestly independently isolated oxygen, called by Priestly "dephlogisticated air" and Scheele "fire air".[26][27]
Joseph Proust proposed the law of definite proportions, which states that elements always combine in small, whole number ratios to form compounds.[28] In 1800, Alessandro Volta devised the first chemical battery, thereby founding the discipline of electrochemistry.[29] In 1803, John Dalton proposed Dalton's Law, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.[30]
Antoine Lavoisier
[編輯]
Although the archives of chemical research draw upon work from ancient Babylonia, Egypt, and especially the Arabs and Persians after Islam, modern chemistry flourished from the time of Antoine Lavoisier, who is regarded as the "father of modern chemistry", particularly for his discovery of the law of conservation of mass, and his refutation of the phlogiston theory of combustion in 1783. (Phlogiston was supposed to be an imponderable substance liberated by flammable materials in burning.) Mikhail Lomonosov independently established a tradition of chemistry in Russia in the 18th century.[來源請求] Lomonosov also rejected the phlogiston theory, and anticipated the kinetic theory of gases.[來源請求] He regarded heat as a form of motion, and stated the idea of conservation of matter.
The vitalism debate and organic chemistry
[編輯]After the nature of combustion (see oxygen) was settled, another dispute, about vitalism and the essential distinction between organic and inorganic substances, was revolutionized by Friedrich Wöhler's accidental synthesis of urea from inorganic substances in 1828. Never before had an organic compound been synthesized from inorganic material.[來源請求] This opened a new research field in chemistry, and by the end of the 19th century, scientists were able to synthesize hundreds of organic compounds. The most important among them are mauve, magenta, and other synthetic dyes, as well as the widely used drug aspirin. The discovery also contributed greatly to the theory of isomerism.[來源請求]
Disputes about atomism after Lavoisier
[編輯]
Throughout the 19th century, chemistry was divided between those who followed the atomic theory of John Dalton and those who did not, such as Wilhelm Ostwald and Ernst Mach.[31] Although such proponents of the atomic theory as Amedeo Avogadro and Ludwig Boltzmann made great advances in explaining the behavior of gases, this dispute was not finally settled until Jean Perrin's experimental investigation of Einstein's atomic explanation of Brownian motion in the first decade of the 20th century.[31]
Well before the dispute had been settled, many had already applied the concept of atomism to chemistry. A major example was the ion theory of Svante Arrhenius which anticipated ideas about atomic substructure that did not fully develop until the 20th century. Michael Faraday was another early worker, whose major contribution to chemistry was electrochemistry, in which (among other things) a certain quantity of electricity during electrolysis or electrodeposition of metals was shown to be associated with certain quantities of chemical elements, and fixed quantities of the elements therefore with each other, in specific ratios.[來源請求] These findings, like those of Dalton's combining ratios, were early clues to the atomic nature of matter.
The periodic table
[編輯]
For many decades, the list of known chemical elements had been steadily increasing. A great breakthrough in making sense of this long list (as well as in understanding the internal structure of atoms as discussed below) was Dmitri Mendeleev and Lothar Meyer's development of the periodic table, and particularly Mendeleev's use of it to predict the existence and the properties of germanium, gallium, and scandium, which Mendeleev called ekasilicon, ekaaluminium, and ekaboron respectively. Mendeleev made his prediction in 1870; gallium was discovered in 1875, and was found to have roughly the same properties that Mendeleev predicted for it.[來源請求]
The modern definition of chemistry
[編輯]Classically, before the 20th century, chemistry was defined as the science of the nature of matter and its transformations. It was therefore clearly distinct from physics which was not concerned with such dramatic transformation of matter. Moreover, in contrast to physics, chemistry was not using much of mathematics. Even some were particularly reluctant to using mathematics within chemistry. For example, Auguste Comte wrote in 1830:
- Every attempt to employ mathematical methods in the study of chemical questions must be considered profoundly irrational and contrary to the spirit of chemistry.... if mathematical analysis should ever hold a prominent place in chemistry -- an aberration which is happily almost impossible -- it would occasion a rapid and widespread degeneration of that science.
However, in the second part of the 19th century, the situation changed and August Kekule wrote in 1867:
- I rather expect that we shall someday find a mathematico-mechanical explanation for what we now call atoms which will render an account of their properties.
After the discovery by Ernest Rutherford and Niels Bohr of the atomic structure in 1912, and by Marie and Pierre Curie of radioactivity, scientists had to change their viewpoint on the nature of matter. The experience acquired by chemists was no longer pertinent to the study of the whole nature of matter but only to aspects related to the electron cloud surrounding the atomic nuclei and the movement of the latter in the electric field induced by the former (see Born-Oppenheimer approximation). The range of chemistry was thus restricted to the nature of matter around us in conditions which are not too far from standard conditions for temperature and pressure and in cases where the exposure to radiation is not too different from the natural microwave, visible or UV radiations on Earth. Chemistry was therefore re-defined as the science of matter that deals with the composition, structure, and properties of substances and with the transformations that they undergo.[來源請求] However the meaning of matter used here relates explicitly to substances made of atoms and molecules, disregarding the matter within the atomic nuclei and its nuclear reaction or matter within highly ionized plasmas. Nevertheless the field of chemistry is still, on our human scale, very broad and the claim that chemistry is everywhere is accurate.
Quantum chemistry
[編輯]Some view the birth of quantum chemistry in the discovery of the Schrödinger equation and its application to the hydrogen atom in 1926.[來源請求] However, the 1927 article of Walter Heitler and Fritz London[32] is often recognised as the first milestone in the history of quantum chemistry.[33] This is the first application of quantum mechanics to the diatomic hydrogen molecule, and thus to the phenomenon of the chemical bond. In the following years much progress was accomplished by Edward Teller, Robert S. Mulliken, Max Born, J. Robert Oppenheimer, Linus Pauling, Erich Hückel, Douglas Hartree, Vladimir Aleksandrovich Fock, to cite a few.[來源請求]
Still, skepticism remained as to the general power of quantum mechanics applied to complex chemical systems.[來源請求] The situation around 1930 is described by Paul Dirac:[34]
"The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known, and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble. It therefore becomes desirable that approximate practical methods of applying quantum mechanics should be developed, which can lead to an explanation of the main features of complex atomic systems without too much computation. Hence the quantum mechanical methods developed in the 1930s and 1940s are often referred to as theoretical molecular or atomic physics to underline the fact that they were more the application of quantum mechanics to chemistry and spectroscopy than answers to chemically relevant questions."
In the 1940s many physicists turned from molecular or atomic physics to nuclear physics (like J. Robert Oppenheimer or Edward Teller). In 1951, a milestone article in quantum chemistry is the seminal paper of Clemens C. J. Roothaan on Roothaan equations.[35] It opened the avenue to the solution of the self-consistent field equations for small molecules like hydrogen or nitrogen. Those computations were performed with the help of tables of integrals which were computed on the most advanced computers of the time.[來源請求]
Molecular biology and biochemistry
[編輯]By the mid 20th century, in principle, the integration of physics and chemistry was extensive, with chemical properties explained as the result of the electronic structure of the atom; Linus Pauling's book on The Nature of the Chemical Bond used the principles of quantum mechanics to deduce bond angles in ever-more complicated molecules. However, though some principles deduced from quantum mechanics were able to predict qualitatively some chemical features for biologically relevant molecules, they were, till the end of the 20th century, more a collection of rules, observations, and recipes than rigorous ab initio quantitative methods.[來源請求]
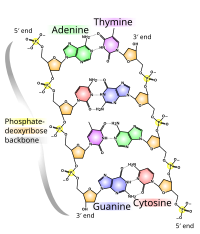
This heuristic approach triumphed in 1953 when James Watson and Francis Crick deduced the double helical structure of DNA by constructing models constrained by and informed by the knowledge of the chemistry of the constituent parts and the X-ray diffraction patterns obtained by Rosalind Franklin.[36] This discovery lead to an explosion of research into the biochemistry of life.
In the same year, the Miller-Urey experiment demonstrated that basic constituents of protein, simple amino acids, could themselves be built up from simpler molecules in a simulation of primordial processes on Earth. Though many questions remain about the true nature of the origin of life, this was the first attempt by chemists to study hypothetical processes in the laboratory under controlled conditions.[來源請求]
In 1983 Kary Mullis devised a method for the in-vitro amplification of DNA, known as the polymerase chain reaction (PCR), which revolutionized the chemical processes used in the laboratory to manipulate it. PCR could be used to synthesize specific pieces of DNA and made possible the sequencing of DNA of organisms, which culminated in the huge human genome project.
An important piece in the double helix puzzle was solved by one of Pauling's student Matthew Meselson and Frank Stahl, the result of their collaboration (Meselson-Stahl experiment) has been called as "the most beatiful experiment in biology".
They used a centrifugation technique that sorted molecules according to differences in weight. Because nitrogen atoms are a component of DNA, they were labelled and therefore tracked in replication in bacteria.
Chemical industry
[編輯]The later part of the nineteenth century saw a huge increase in the exploitation of petroleum extracted from the earth for the production of a host of chemicals and largely replaced the use of whale oil, coal tar and naval stores used previously. Large scale production and refinement of petroleum provided feedstocks for liquid fuels such as gasoline and diesel, solvents, lubricants, asphalt, waxes, and for the production of many of the common materials of the modern world, such as synthetic fibers, plastics, paints, detergents, pharmaceuticals, adhesives and ammonia as fertilizer and for other uses. Many of these required new catalysts and the utilization of chemical engineering for their cost-effective production.
In the mid-twentieth century, control of the electronic structure of semiconductor materials was made precise by the creation of large ingots of extremely pure single crystals of silicon and germanium. Accurate control of their chemical composition by doping with other elements made the production of the solid state transistor in 1951 and made possible the production of tiny integrated circuits for use in electronic devices, especially computers.
See also
[編輯]Histories and timelines
[編輯]- Atomic theory
- History of chromatography
- History of electrochemistry
- History of the molecule
- History of molecular biology
- History of the periodic table
- History of physics
- History of science and technology
- History of thermodynamics
- History of energy
- Discoveries of the chemical elements
- Timeline of chemistry
- Timeline of materials technology
- Timeline of microphysics
- Timeline of thermodynamics, statistical mechanics, and random processes
- List of years in science
- Nobel Prize in chemistry
Chemists
[編輯]listed chronologically:
- List of chemists
- Joseph Black, 1728-1799
- Joseph Priestley, 1733-1804
- Carl Wilhelm Scheele, 1742-1786
- Alessandro Volta, 1745-1827
- Jacques Charles, 1746-1823
- Claude Louis Berthollet, 1748-1822
- Joseph-Louis Gay-Lussac, 1778-1850
- Humphry Davy, 1778-1829
- Jöns Jakob Berzelius, inventor of modern chemical notation, 1779-1848
- Justus von Liebig, 1803-1873
- Louis Pasteur, 1822-1895
- Stanislao Cannizzaro, 1826-1910
- Friedrich August Kekulé von Stradonitz, 1829-1896
- Willard Gibbs, 1839-1903
- J. H. van 't Hoff, 1852-1911
- Marie Curie, 1867-1934
- Victor Grignard, 1871-1935
- Gilbert N. Lewis, 1875-1946
- Otto Hahn, 1879-1968
- Irving Langmuir, 1881-1957
- Walther Nernst, 1864-1941
- Linus Pauling, 1901-1994
- Glenn T. Seaborg, 1912-1999
- Frederick Sanger, 1918-
- Rudolph A. Marcus, 1923-
- Harold Kroto, 1939-
- Richard Smalley, 1943-2005
- Peter Atkins, 1940-
Notes
[編輯]- ^ Selected Classic Papers from the History of Chemistry
- ^ 2.0 2.1
Will Durant (1935), Our Oriental Heritage:
"Two systems of Hindu thought propound physical theories suggestively similar to those of Greece. Kanada, founder of the Vaisheshika philosophy, held that the world was composed of atoms as many in kind as the various elements. The Jains more nearly approximated to Democritus by teaching that all atoms were of the same kind, producing different effects by diverse modes of combinations. Kanada believed light and heat to be varieties of the same substance; Udayana taught that all heat comes from the sun; and Vachaspati, like Newton, interpreted light as composed of minute particles emitted by substances and striking the eye."
- ^ Lucretius. de Rerum Natura (On the Nature of Things). The Internet Classics Archive. Massachusetts Institute of Technology. 50 BCE [2007-01-09].
- ^ Simpson, David. Lucretius (c. 99 - c. 55 BCE). The Internet History of Philosophy. 29 June 2005 [2007-01-09].
- ^ Will Durant wrote in The Story of Civilization I: Our Oriental Heritage:
"Something has been said about the chemical excellence of cast iron in ancient India, and about the high industrial development of the Gupta times, when India was looked to, even by Imperial Rome, as the most skilled of the nations in such chemical industries as dyeing, tanning, soap-making, glass and cement... By the sixth century the Hindus were far ahead of Europe in industrial chemistry; they were masters of calcinations, distillation, sublimation, steaming, fixation, the production of light without heat, the mixing of anesthetic and soporific powders, and the preparation of metallic salts, compounds and alloys. The tempering of steel was brought in ancient India to a perfection unknown in Europe till our own times; King Porus is said to have selected, as a specially valuable gift from Alexander, not gold or silver, but thirty pounds of steel. The Moslems took much of this Hindu chemical science and industry to the Near East and Europe; the secret of manufacturing "Damascus" blades, for example, was taken by the Arabs from the Persians, and by the Persians from India."
- ^ Brock, William H. The Fontana History of Chemistry. London, England: Fontana Press. 1992: 32–33.
- ^ Brock, William H. The Fontana History of Chemistry. London, England: Fontana Press. 1992.
- ^ The History of Ancient Chemistry
- ^ Derewenda, Zygmunt S., On wine, chirality and crystallography, Acta Crystallographica Section A: Foundations of Crystallography, 2007, 64: 246–258 [247], doi:10.1107/S0108767307054293
- ^ John Warren (2005). "War and the Cultural Heritage of Iraq: a sadly mismanaged affair", Third World Quarterly, Volume 26, Issue 4 & 5, p. 815-830.
- ^ Dr. A. Zahoor (1997), JABIR IBN HAIYAN (Jabir), University of Indonesia
- ^ Paul Vallely, How Islamic inventors changed the world, The Independent
- ^ Kraus, Paul, Jâbir ibn Hayyân, Contribution à l'histoire des idées scientifiques dans l'Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque,. Cairo (1942-1943). Repr. By Fuat Sezgin, (Natural Sciences in Islam. 67-68), Frankfurt. 2002:
「To form an idea of the historical place of Jabir’s alchemy and to tackle the problem of its sources, it is advisable to compare it with what remains to us of the alchemical literature in the Greek language. One knows in which miserable state this literature reached us. Collected by Byzantine scientists from the tenth century, the corpus of the Greek alchemists is a cluster of incoherent fragments, going back to all the times since the third century until the end of the Middle Ages.」
「The efforts of Berthelot and Ruelle to put a little order in this mass of literature led only to poor results, and the later researchers, among them in particular Mrs. Hammer-Jensen, Tannery, Lagercrantz , von Lippmann, Reitzenstein, Ruska, Bidez, Festugiere and others, could make clear only few points of detail…
The study of the Greek alchemists is not very encouraging. An even surface examination of the Greek texts shows that a very small part only was organized according to true experiments of laboratory: even the supposedly technical writings, in the state where we find them today, are unintelligible nonsense which refuses any interpretation.
It is different with Jabir’s alchemy. The relatively clear description of the processes and the alchemical apparatuses, the methodical classification of the substances, mark an experimental spirit which is extremely far away from the weird and odd esotericism of the Greek texts. The theory on which Jabir supports his operations is one of clearness and of an impressive unity. More than with the other Arab authors, one notes with him a balance between theoretical teaching and practical teaching, between the `ilm and the `amal. In vain one would seek in the Greek texts a work as systematic as that which is presented for example in the Book of Seventy.」
(cf. Ahmad Y Hassan. A Critical Reassessment of the Geber Problem: Part Three. [2008-08-09].)
- ^ Will Durant (1980). The Age of Faith (The Story of Civilization, Volume 4), p. 162-186. Simon & Schuster. ISBN 0671012002.
- ^ Research Committee of Strasburg University, Imam Jafar Ibn Muhammad As-Sadiq A.S. The Great Muslim Scientist and Philosopher, translated by Kaukab Ali Mirza, 2000. Willowdale Ont. ISBN 0969949014.
- ^ Felix Klein-Frank (2001), "Al-Kindi", in Oliver Leaman & Hossein Nasr, History of Islamic Philosophy, p. 174. London: Routledge.
- ^ Michael E. Marmura (1965). "An Introduction to Islamic Cosmological Doctrines. Conceptions of Nature and Methods Used for Its Study by the Ikhwan Al-Safa'an, Al-Biruni, and Ibn Sina by Seyyed Hossein Nasr", Speculum 40 (4), p. 744-746.
- ^ Robert Briffault (1938). The Making of Humanity, p. 196-197.
- ^ Farid Alakbarov (Summer 2001). A 13th-Century Darwin? Tusi's Views on Evolution, Azerbaijan International 9 (2).
- ^ G. Stolyarov II (2002), "Rhazes: The Thinking Western Physician", The Rational Argumentator, Issue VI.
- ^ Asarnow, Herman. Sir Francis Bacon: Empiricism. An Image-Oriented Introduction to Backgrounds for English Renaissance Literature. University of Portland. 2005-08-08 [2007-02-22].
- ^ Crosland, M.P. (1959). "The use of diagrams as chemical 'equations' in the lectures of William Cullen and Joseph Black." Annals of Science, Vol 15, No. 2, Jun.
- ^ Robert Boyle
- ^ Lavoisier, Antoine (1743-1794) -- from Eric Weisstein's World of Scientific Biography, ScienceWorld
- ^ Cooper, Alan. Joseph Black. History of Glasgow University Chemistry Department. University of Glasgow Department of Chemistry. 1999 [2006-02-23].
- ^ Joseph Priestley. Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005 [2007-02-22].
- ^ Carl Wilhelm Scheele. History of Gas Chemistry. Center for Microscale Gas Chemistry, Creighton University. 2005-09-11 [2007-02-23].
- ^ Proust, Joseph Louis (1754-1826). 100 Distinguished Chemists. European Association for Chemical and Molecular Science. 2005 [2007-02-23].
- ^ Inventor Alessandro Volta Biography. The Great Idea Finder. The Great Idea Finder. 2005 [2007-02-23].
- ^ John Dalton. Chemical Achievers: The Human Face of Chemical Sciences. Chemical Heritage Foundation. 2005 [2007-02-22].
- ^ 31.0 31.1 Pullman, Bernard. The Atom in the History of Human Thought. 由Reisinger, Axel翻譯. USA: Oxford University Press Inc. 2004.
- ^ W. Heitler and F. London, Wechselwirkung neutraler Atome und Homöopolare Bindung nach der Quantenmechanik, Z. Physik, 44, 455 (1927).
- ^ Quantum chemistry
- ^ P.A.M. Dirac, Quantum Mechanics of Many-Electron Systems, Proc. R. Soc. London, A 123, 714 (1929).
- ^ C.C.J. Roothaan, A Study of Two-Center Integrals Useful in Calculations on Molecular Structure, J. Chem. Phys., 19, 1445 (1951).
- ^ Watson, J. and Crick, F., "Molecular Structure of Nucleic Acids" Nature, April 25, 1953, p 737–8
References
[編輯]- Selected classic papers from the history of chemistry
- Biographies of chemists
- Eric R. Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006.
External links
[編輯]- ChemisLab - Chemists of the Past
- SHAC: Society for the History of Alchemy and Chemistry
