User:Kaguya-Taketori/RNA


核糖核酸(英語:Ribonucleic acid),簡稱RNA,係一類由核糖核苷酸通過3',5'-磷酸二酯鍵聚合而成的線性大分子[1]。自然界中的RNA通常是單鏈的,且RNA中最基本的四種鹼基爲A(腺嘌呤)、U(尿嘧啶)、G(鳥嘌呤)、C(胞嘧啶)[註 1],相對的,與RNA同爲核酸的DNA通常是雙鏈分子,且含有的含氮鹼基爲A(腺嘌呤)、T(胸腺嘧啶)、G(鳥嘌呤)、C(胞嘧啶)四種。
RNA有着多種多樣的功能,可在遺傳編碼、翻譯、調控、基因表達等過程中發揮作用。按RNA的功能,可將RNA分爲多種類型。比如,在細胞生物中,mRNA(信使RNA)爲遺傳信息的傳遞者,它能夠指導蛋白質的合成。因爲mRNA有編碼蛋白質的能力,它又被稱爲編碼RNA。而其他沒有編碼蛋白質能力的RNA則被稱爲非編碼RNA(ncRNA)。它們或通過催化生化反應,或通過調控或參與基因表達過程發揮相應的生物學功能。比如,tRNA(轉運RNA)在翻譯過程中起轉運RNA的作用,rRNA(核糖體RNA)於翻譯過程中起催化肽鏈形成的作用,sRNA(小RNA)起到調控基因表達的作用。此外,RNA病毒甚至以RNA作爲它們的遺傳物質。
RNA通常由DNA通過轉錄生成。RNA在細胞中廣泛分佈,真核生物的細胞核、細胞質、粒線體中都有RNA[2]:36。
與DNA的比較
[编辑]
與DNA相似,大部分有生物活性的RNA,包括mRNA、tRNA、rRNA、snRNA,以及其他一些非編碼RNA,雖然是單鏈,但含有自我互補的序列,能使得它們能進行摺疊[7],形成互補雙鏈結構(莖)。對RNA的分析表明,它們有着相對更複雜的結構。和DNA不同,RNA的二級結構並不是單純的雙螺旋,而由一系列短的二級結構構成。通過這些短的二級結構的組合,RNA甚至可以擁有與蛋白質相似的結構,並像酶那樣催化化學反應(這樣的RNA被稱爲核酶)[8]。比如,對核糖體進行分析表明,其催化成肽反應的活性位點完全由RNA構成[9]。
結構
[编辑]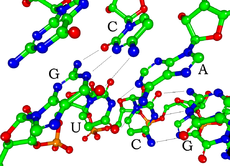
RNA的單體爲核糖核苷酸,其中的戊糖爲核糖,依系統命名法可將其中的碳原子從1'編號至5'。含氮鹼基與1'碳原子相連。RNA中最基本的四種鹼基分別爲A(腺嘌呤)、U(尿嘧啶)、G(鳥嘌呤)、C(胞嘧啶)。其中,腺嘌呤和鳥嘌呤爲雙環的嘌呤,尿嘧啶和胞嘧啶爲單環的嘧啶。磷酸基團與一個核糖殘基的3'碳原子相連,與下一個核糖核苷酸的5'碳原子相連。磷酸基團在生理pH下,並不都能帶上負電荷,因而RNA在生理條件下是帶電荷分子(聚陰離子)。C和G、U和A、G和U之間能夠形成氫鍵[10]。不過,鹼基之間也可能發生其他一些相互作用。比如,在一個凸出部分中,一群腺嘌呤可以互相連接[11],GNRA四環中有一個G-A鹼基對[10]。
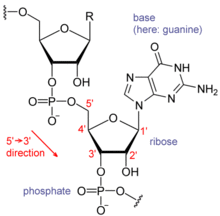
核糖的2位碳上連有羥基爲RNA的一個重要結構特點。這類羥基使得RNA雙鏈的結構應與A型構象最接近[12],不過,在單鏈的某些二核苷酸環境下,也有極小的可能形成DNA最常見的B型螺旋構象[13]。A型構象使得RNA雙鏈的大溝狹窄而深,小溝淺而寬[14]。在RNA分子的構象高度可變區域(即不生成雙鏈結構的區域),2'-OH還能攻擊附近的磷酸二酯鍵,使得核糖-磷酸鏈斷裂[15]。
通過轉錄,僅僅能使RNA鏈上帶A、U、G、C四種含氮鹼基[16]不過,轉錄後修飾能夠通過多種途徑對RNA進行改造。比如,轉錄後修飾能夠將稀有鹼基假尿嘧啶(Ψ)加到RNA鏈上。假尿嘧啶與核糖之間的化學鍵是C-C鍵而不是尿嘧啶(U)的C-N鍵。胸腺嘧啶加到RNA鏈上的情形也很常見(最典型的例子是tRNA的TΨC環)[17]。另外,次黃嘌呤也是一種常見的稀有鹼基。次黃嘌呤爲腺嘌呤的脫氨產物,含有次黃嘌呤的核苷被稱爲肌苷(I)。在基因編碼的擺動假說中,肌苷有重要的作用[18]。
除以上列出的之外,經過編輯的核苷還有100多種[19]。由修飾引發的結構性變化在tRNA中最爲明顯[20],假尿嘧啶與經常在rRNA中出現2'-甲氧基核糖是最常見的修飾產物[21]。這些修飾的具體作用還沒有完全闡明。不過,值得注意的是,在rRNA中,許多的轉錄後修飾發生在高度功能化的區域,比如肽基轉移酶催化中心以及亞基結合部位,似乎說明轉錄後修飾對RNA發揮正常功能來說相當重要[22]。
具有催化功能的單鏈RNA分子,和蛋白質相類似,需要特殊的RNA三級結構。通過分子內氫鍵形成的二級結構原件構成了三級結構的框架。二級結構形成了許多可識別的「結構域」——比如莖環結構、膨大結構(bulges)、內環結構[23]。因爲RNA分子帶電荷,不少二級結構和三級結構需要Mg2+等金屬離子來進行穩定[24]。
自然界中的RNA均是由D-核糖核苷酸聚合而成的D-RNA。使用L-核糖核苷酸則可合成L-RNA。L-RNA對RNA酶的耐受力要強得多[25]。
合成與修飾
[编辑]RNA的合成一般由RNA聚合酶催化。RNA聚合酶以DNA爲模板,通過轉錄合成RNA。轉錄起始於RNA聚合酶與啓動子的結合(啓動子一般位於基因的上游)。因爲RNA聚合酶自帶解旋酶活性,僅依靠RNA聚合酶即可實現DNA雙鏈的解開。轉錄過程中,RNA聚合酶以3'端至5'端的方向讀取DNA模板鏈,並以5'端到3'端的方向合成與之反向平行互補的RNA鏈。轉錄的終止由終止子介導。原核生物的終止子有兩類:簡單終止子與ρ因子依賴性終止子。簡單終止子僅靠RNA形成二級結構即可終止轉錄,而後者在ρ因子的作用下才可以使轉錄終止。真核生物的轉錄終止則與轉錄後修飾密切相關[2]:323[26][27]:531-535。
在真核生物中,RNA的初始轉錄物通常會經過轉錄後修飾。比如,真核生物的mRNA大都會被加上Poly(A)尾(多腺嘌呤尾巴)以及5'端帽,mRNA前體中含有的內含子序列也會被剪接體切除[27]:580-581。
一些RNA是由RNA複製酶(RNA依賴性RNA聚合酶)以RNA爲模板催化合成的。比方說,RNA病毒通過RNA複製酶複製其遺傳物質[28]。另外,RNA複製酶亦參與了眾多生物體的RNA干涉過程[29]。
RNA的種類
[编辑]總覽
[编辑]
mRNA爲攜帶遺傳信息的RNA,它所攜帶的遺傳信息由核糖體讀取,並指導蛋白質合成。mRNA的序列決定了轉譯出的多肽鏈的氨基酸序列[30]。然而,絕大部分的RNA並不編碼蛋白質(真核生物有大約97%的轉錄產物不編碼蛋白質[31][32][33][34])。這樣的RNA被稱爲非編碼RNA(ncRNA)。它們可能由相應的基因編碼(這樣的基因叫RNA基因),也可以由mRNA的內含子演化而成[35]。最常見的非編碼RNA當屬tRNA和rRNA,它們都參與轉譯過程[6]。非編碼RNA亦參與基因表達調控(比如轉錄後修飾等過程)。也有一些RNA具有催化化學反應的作用,比如對RNA進行剪接[36]、催化多肽鏈的合成[9],這類RNA即爲核酶。
按長度分
[编辑]根據RNA鏈的長度,可將RNA分爲小RNA(sRNA)和長鏈RNA(long RNA)[37]。一般地,小RNA是指長度小於200nt的RNA,長鏈RNA是指長度大於200nt的RNA[38]。長鏈RNA,也叫大RNA(large RNA),主要包括長鏈非編碼RNA(lncRNA)和mRNA。小RNA主要包括5.8S rRNA、5S rRNA、tRNA、miRNA(微RNA)、siRNA(小干擾RNA)、snoRNA(小核仁RNA)、snRNA(小核RNA)、scRNA(細胞質小RNA)[2]:49-50。
在轉譯過程中
[编辑]mRNA攜帶遺傳信息,核糖體會讀取其所攜帶的遺傳信息,並合成相應的蛋白質。核糖體以三聯密碼子的形式讀取mRNA上的遺傳信息,每一個三聯密碼子對應一個多肽鏈的氨基酸(終止密碼子除外)。在真核細胞中,轉錄出的mRNA前體需要經過加工才能成爲成熟的mRNA。加工過程會切除掉mRNA前體的內含子區(不編碼的區域)。mRNA隨後會從細胞核中轉移到細胞質中,並與核糖體結合,在tRNA的幫助下,由核糖體讀取其遺傳信息並合成蛋白質。原核生物無成形細胞核,擬核和細胞質無明確界限,在轉錄還未完成時,核糖體就已經與mRNA結合,並開始轉譯過程,而且轉錄完成後,mRNA就會很快被核酸酶攻擊降解,降解時轉譯過程往往還沒有停止[30]。
tRNA長約80nt,攜帶氨基酸至核糖體上,爲肽鏈的延伸提供底物。一個tRNA有氨基酸結合區域(5' CCA 3')以及可以與對應密碼子互補的反密碼子區[35]。
核糖體RNA構成了核糖體的催化核心。真核生物的核糖體包含18S、5.8S、28S以及5S rRNA(原核生物的核糖體則包含16S、5S、23S rRNA)。真核生物的三種rRNA在核仁中合成,另外一種則在核基質中合成。在細胞質中,rRNA和蛋白質裝配形成一種核糖核蛋白複合物,即核糖體。核糖體與mRNA結合,並催化蛋白質的合成,一條mRNA鏈可同時與多個核糖體結合[30]。典型真核生物中的RNA幾乎都是rRNA。
tmRNA(信使—轉運RNA)在許多細菌和色素體中都有分佈。它標記由缺少終止密碼子的mRNA編碼的蛋白質,使其降解。同時,它還能阻止核糖體的停止[39]。
Regulatory RNAs
[编辑]Several types of RNA can downregulate gene expression by being complementary to a part of an mRNA or a gene's DNA.[40][41] MicroRNAs (miRNA; 21-22 nt) are found in eukaryotes and act through RNA interference (RNAi), where an effector complex of miRNA and enzymes can cleave complementary mRNA, block the mRNA from being translated, or accelerate its degradation.[42][43]
While small interfering RNAs (siRNA; 20-25 nt) are often produced by breakdown of viral RNA, there are also endogenous sources of siRNAs.[44][45] siRNAs act through RNA interference in a fashion similar to miRNAs. Some miRNAs and siRNAs can cause genes they target to be methylated, thereby decreasing or increasing transcription of those genes.[46][47][48] Animals have Piwi-interacting RNAs (piRNA; 29-30 nt) that are active in germline cells and are thought to be a defense against transposons and play a role in gametogenesis.[49][50]
Many prokaryotes have CRISPR RNAs, a regulatory system similar to RNA interference.[51] Antisense RNAs are widespread; most downregulate a gene, but a few are activators of transcription.[52] One way antisense RNA can act is by binding to an mRNA, forming double-stranded RNA that is enzymatically degraded.[53] There are many long noncoding RNAs that regulate genes in eukaryotes,[54] one such RNA is Xist, which coats one X chromosome in female mammals and inactivates it.[55]
An mRNA may contain regulatory elements itself, such as riboswitches, in the 5' untranslated region or 3' untranslated region; these cis-regulatory elements regulate the activity of that mRNA.[56] The untranslated regions can also contain elements that regulate other genes.[57]
In RNA processing
[编辑]
Many RNAs are involved in modifying other RNAs. Introns are spliced out of pre-mRNA by spliceosomes, which contain several small nuclear RNAs (snRNA),[6] or the introns can be ribozymes that are spliced by themselves.[58] RNA can also be altered by having its nucleotides modified to other nucleotides than A, C, G and U. In eukaryotes, modifications of RNA nucleotides are in general directed by small nucleolar RNAs (snoRNA; 60-300 nt),[35] found in the nucleolus and cajal bodies. snoRNAs associate with enzymes and guide them to a spot on an RNA by basepairing to that RNA. These enzymes then perform the nucleotide modification. rRNAs and tRNAs are extensively modified, but snRNAs and mRNAs can also be the target of base modification.[59][60] RNA can also be methylated.[61][62]
RNA genomes
[编辑]Like DNA, RNA can carry genetic information. RNA viruses have genomes composed of RNA that encodes a number of proteins. The viral genome is replicated by some of those proteins, while other proteins protect the genome as the virus particle moves to a new host cell. Viroids are another group of pathogens, but they consist only of RNA, do not encode any protein and are replicated by a host plant cell's polymerase.[63]
In reverse transcription
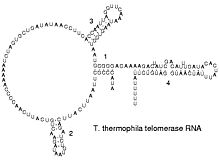
[编辑]Reverse transcribing viruses replicate their genomes by reverse transcribing DNA copies from their RNA; these DNA copies are then transcribed to new RNA. Retrotransposons also spread by copying DNA and RNA from one another,[64] and telomerase contains an RNA that is used as template for building the ends of eukaryotic chromosomes.[65]
Double-stranded RNA
[编辑]Double-stranded RNA (dsRNA) is RNA with two complementary strands, similar to the DNA found in all cells. dsRNA forms the genetic material of some viruses (double-stranded RNA viruses). Double-stranded RNA such as viral RNA or siRNA can trigger RNA interference in eukaryotes, as well as interferon response in vertebrates.[66][67][68][69]
RNA生物學中的重大發現
[编辑]
對RNA的研究催生了許多重大的生物學發現,諾貝爾獎亦數次垂青對RNA進行研究的科學家。1868年,弗雷德里希·米歇爾發現了核酸。因爲他是在細胞核中發現這種物質的,所以,他把這種物質稱爲「核素」(nuclein)[70]。後來,人們發現沒有成形細胞核的原核細胞仍然含有核酸。早在1939年,就有人提出RNA可能參與蛋白質合成[71]。塞韋羅·奧喬亞因發現一種能夠在實驗室中合成RNA的酶而與阿瑟·科恩伯格獲得1959年諾貝爾生理醫學獎[72]。然而,由奧喬亞發現的酶(多核苷酸磷酸化酶)隨後卻被證明是負責RNA降解,而不是RNA合成的。1956年,亞歷山大·里奇和大衛·戴維斯使兩條RNA進行雜交,並製得了第一批RNA晶體。該晶體的結構可通過X射線衍射確定[73]。
1965年,羅伯特·W·霍利測定了長77nt(nt:核苷酸)的酵母菌tRNA的序列[74],這使得他與哈爾·葛賓·科拉納、馬歇爾·沃倫·尼倫伯格獲得了1968年的諾貝爾生理醫學獎。1967年,卡爾·烏斯提出可能存在具有催化功能的RNA。他還認爲,早期的生命(自我複製的分子)可能僅用RNA來進行催化和攜帶遺傳信息(即RNA世界假說)[75][76]。
1970年代早期,發現了逆轉錄病毒以及逆轉錄酶,證明酶可以用RNA爲模板合成DNA(這和通常的遺傳信息傳遞方向相反)。戴維·巴爾的摩、羅納托·杜爾貝科、霍華德·馬丁·特明因此獲得了1975年諾貝爾生理醫學獎。1976年,瓦爾特·费爾斯與他的研究團隊發現了第一段與RNA病毒MS2噬菌體基因組互補的核苷酸序列[77]。
1977年,在哺乳動物病毒以及細胞基因中發現了內含子以及RNA剪接現象。這一發現使得理察·羅伯茨與菲利普·夏普獲得1993年諾貝爾生理醫學獎。核酶最早於1980年代早期發現,使西德尼·奧特曼、托馬斯·切赫兩人獲1989年諾貝爾化學獎。1990年,對矮牽牛花的實驗表明,引入的基因可以沉默植物體原有的基因,這一現象即RNA干擾[78][79]。
1990年左右,發現一種22nt長的RNA在秀麗隱桿線蟲的發育過程中發揮作用,這種RNA即微RNA(microRNA)[80]。對RNA干擾的研究使得安德魯·法厄與克雷格·梅洛獲得2006年生理醫學獎。有趣的是,當年的諾貝爾化學獎亦頒給了對RNA轉錄進行研究的羅傑·科恩伯格。基因調控RNA的發現使得人們開始研發以RNA爲化學本質(比如siRNA(小干擾RNA))的藥物來使得基因沉默[81]。
See also
[编辑]- Biomolecular structure
- DNA, RNA and proteins: The three essential macromolecules of life
- DNA
- History of RNA Biology
- List of RNA Biologists
註釋
[编辑]參考
[编辑]- ^ David Hames & Nigel Hooper. Instant Notes in Biochemistry (導讀本) 3rd edition. 科学出版社. 2005: 193. ISBN 978-7-03-025218-0.
- ^ 2.0 2.1 2.2 查錫良 藥立波. 生物化學與分子生物學 第八版. 人民衛生出版社. 2013. ISBN 978-7-117-17214-1.
- ^ RNA: The Versatile Molecule. University of Utah. 2015.
- ^ Nucleotides and Nucleic Acids (PDF). University of California, Los Angeles.
- ^ R.N. Shukla. Analysis of Chromosomes. ISBN 9789384568177.
- ^ 6.0 6.1 6.2 Berg JM; Tymoczko JL; Stryer L. Biochemistry 5th. WH Freeman and Company. 2002: 118–19, 781–808. ISBN 0-7167-4684-0. OCLC 179705944.
- ^ I. Tinoco & C. Bustamante. How RNA folds. J. Mol. Biol. 1999, 293 (2): 271–281. PMID 10550208. doi:10.1006/jmbi.1999.3001.
- ^ Higgs PG. RNA secondary structure: physical and computational aspects. Quarterly Reviews of Biophysics. 2000, 33 (3): 199–253. PMID 11191843. doi:10.1017/S0033583500003620.
- ^ 9.0 9.1 Nissen P; Hansen J; Ban N; Moore PB; Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000, 289 (5481): 920–30. Bibcode:2000Sci...289..920N. PMID 10937990. doi:10.1126/science.289.5481.920.
- ^ 10.0 10.1 Lee JC; Gutell RR. Diversity of base-pair conformations and their occurrence in rRNA structure and RNA structural motifs. J. Mol. Biol. 2004, 344 (5): 1225–49. PMID 15561141. doi:10.1016/j.jmb.2004.09.072.
- ^ Barciszewski J; Frederic B; Clark C. RNA biochemistry and biotechnology. Springer. 1999: 73–87. ISBN 0-7923-5862-7. OCLC 52403776.
- ^ Salazar M; Fedoroff OY; Miller JM; Ribeiro NS; Reid BR. The DNA strand in DNAoRNA hybrid duplexes is neither B-form nor A-form in solution. Biochemistry. 1992, 32 (16): 4207–15. PMID 7682844. doi:10.1021/bi00067a007.
- ^ Sedova A; Banavali NK. RNA approaches the B-form in stacked single strand dinucleotide contexts. Biopolymers. 2016, 105 (2): 65–82. PMID 26443416. doi:10.1002/bip.22750.
- ^ Hermann T; Patel DJ. RNA bulges as architectural and recognition motifs. Structure. 2000, 8 (3): R47–R54. PMID 10745015. doi:10.1016/S0969-2126(00)00110-6.
- ^ Mikkola S; Stenman E; Nurmi K; Yousefi-Salakdeh E; Strömberg R; Lönnberg H. The mechanism of the metal ion promoted cleavage of RNA phosphodiester bonds involves a general acid catalysis by the metal aquo ion on the departure of the leaving group. Perkin transactions 2. 1999, (8): 1619–26. doi:10.1039/a903691a.
- ^ Jankowski JAZ; Polak JM. Clinical gene analysis and manipulation: Tools, techniques and troubleshooting. Cambridge University Press. 1996: 14. ISBN 0-521-47896-0. OCLC 33838261.
- ^ Yu Q; Morrow CD. Identification of critical elements in the tRNA acceptor stem and TΨC loop necessary for human immunodeficiency virus type 1 infectivity. J Virol. 2001, 75 (10): 4902–6. PMC 114245
 . PMID 11312362. doi:10.1128/JVI.75.10.4902-4906.2001.
. PMID 11312362. doi:10.1128/JVI.75.10.4902-4906.2001.
- ^ Elliott MS; Trewyn RW. Inosine biosynthesis in transfer RNA by an enzymatic insertion of hypoxanthine. J. Biol. Chem. 1983, 259 (4): 2407–10. PMID 6365911.
- ^ Cantara, WA; Crain, PF; Rozenski, J; McCloskey, JA; Harris, KA; Zhang, X; Vendeix, FA; Fabris, D; Agris, PF. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Research. January 2011, 39 (Database issue): D195–201. PMC 3013656
 . PMID 21071406. doi:10.1093/nar/gkq1028.
. PMID 21071406. doi:10.1093/nar/gkq1028.
- ^ Söll D; RajBhandary U. TRNA: Structure, biosynthesis, and function. ASM Press. 1995: 165. ISBN 1-55581-073-X. OCLC 183036381.
- ^ Kiss T. Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs. The EMBO Journal. 2001, 20 (14): 3617–22. PMC 125535
 . PMID 11447102. doi:10.1093/emboj/20.14.3617.
. PMID 11447102. doi:10.1093/emboj/20.14.3617.
- ^ King TH; Liu B; McCully RR; Fournier MJ. Ribosome structure and activity are altered in cells lacking snoRNPs that form pseudouridines in the peptidyl transferase center. Molecular Cell. 2002, 11 (2): 425–35. PMID 12620230. doi:10.1016/S1097-2765(03)00040-6.
- ^ Mathews DH; Disney MD; Childs JL; Schroeder SJ; Zuker M; Turner DH. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. USA. 2004, 101 (19): 7287–92. Bibcode:2004PNAS..101.7287M. PMC 409911
 . PMID 15123812. doi:10.1073/pnas.0401799101.
. PMID 15123812. doi:10.1073/pnas.0401799101.
- ^ Tan ZJ; Chen SJ. Salt dependence of nucleic acid hairpin stability. Biophys. J. 2008, 95 (2): 738–52. Bibcode:2008BpJ....95..738T. PMC 2440479
 . PMID 18424500. doi:10.1529/biophysj.108.131524.
. PMID 18424500. doi:10.1529/biophysj.108.131524.
- ^ Vater A; Klussmann S. Turning mirror-image oligonucleotides into drugs: the evolution of Spiegelmer therapeutics. Drug Discovery Today. January 2015, 20 (1): 147–155. PMID 25236655. doi:10.1016/j.drudis.2014.09.004.
- ^ Nudler E; Gottesman ME. Transcription termination and anti-termination in E. coli. Genes to Cells. 2002, 7 (8): 755–68. PMID 12167155. doi:10.1046/j.1365-2443.2002.00563.x.
- ^ 27.0 27.1 Jocelyn E.KREBS; et al. Gene XI. JONES&BARTLETT LEARNING(高等教育出版社出版). 2014. ISBN 978-7-04-039649-2.
- ^ Jeffrey L Hansen; Alexander M Long; Steve C Schultz. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure. 1997, 5 (8): 1109–22. PMID 9309225. doi:10.1016/S0969-2126(97)00261-X.
- ^ Ahlquist P. RNA-Dependent RNA Polymerases, Viruses, and RNA Silencing. Science. 2002, 296 (5571): 1270–73. Bibcode:2002Sci...296.1270A. PMID 12016304. doi:10.1126/science.1069132.
- ^ 30.0 30.1 30.2 Cooper GC; Hausman RE. The Cell: A Molecular Approach 3rd. Sinauer. 2004: 261–76, 297, 339–44. ISBN 0-87893-214-3. OCLC 174924833.
- ^ Mattick JS; Gagen MJ. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol. Biol. Evol. 1 September 2001, 18 (9): 1611–30. PMID 11504843. doi:10.1093/oxfordjournals.molbev.a003951.
- ^ Mattick, JS. Noncoding RNAs: the architects of eukaryotic complexity. EMBO Reports. 2001, 2 (11): 986–91. PMC 1084129
 . PMID 11713189. doi:10.1093/embo-reports/kve230.
. PMID 11713189. doi:10.1093/embo-reports/kve230.
- ^ Mattick JS. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms (PDF). BioEssays. October 2003, 25 (10): 930–9. PMID 14505360. doi:10.1002/bies.10332.
- ^ Mattick JS. The hidden genetic program of complex organisms. Scientific American. October 2004, 291 (4): 60–7. PMID 15487671. doi:10.1038/scientificamerican1004-60. (原始内容存档于February 8, 2015).
- ^ 35.0 35.1 35.2 Wirta W. Mining the transcriptome – methods and applications. Stockholm: School of Biotechnology, Royal Institute of Technology. 2006. ISBN 91-7178-436-5. OCLC 185406288.
- ^ Rossi JJ. Ribozyme diagnostics comes of age. Chemistry & Biology. 2004, 11 (7): 894–95. PMID 15271347. doi:10.1016/j.chembiol.2004.07.002.
- ^ Storz, G. An expanding universe of noncoding RNAs.. Science. 17 May 2002, 296 (5571): 1260–3. PMID 12016301. doi:10.1126/science.1072249.
- ^ Fatica, A; Bozzoni, I. Long non-coding RNAs: new players in cell differentiation and development.. Nature Reviews Genetics. January 2014, 15 (1): 7–21. PMID 24296535. doi:10.1038/nrg3606.
- ^ Gueneau de Novoa P, Williams KP; Williams. The tmRNA website: reductive evolution of tmRNA in plastids and other endosymbionts. Nucleic Acids Res. 2004, 32 (Database issue): D104–8. PMC 308836
 . PMID 14681369. doi:10.1093/nar/gkh102.
. PMID 14681369. doi:10.1093/nar/gkh102.
- ^ Carthew, RW; Sontheimer, EJ. Origins and Mechanisms of miRNAs and siRNAs.. Cell. February 2009, 136: 642–55 [2009-02-20]. PMC 2675692
 . PMID 19239886. doi:10.1016/j.cell.2009.01.035.
. PMID 19239886. doi:10.1016/j.cell.2009.01.035.
- ^ Liang, Kung-Hao; Yeh, Chau-Ting. A gene expression restriction network mediated by sense and antisense Alu sequences located on protein-coding messenger RNAs.. BMC Genomics. 2013, 14: 325 [2013-05-11]. PMC 3655826
 . PMID 23663499. doi:10.1186/1471-2164-14-325.
. PMID 23663499. doi:10.1186/1471-2164-14-325.
- ^ Wu L; Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol. Cell. January 2008, 29 (1): 1–7. PMID 18206964. doi:10.1016/j.molcel.2007.12.010.
- ^ Matzke MA; Matzke AJM. Planting the seeds of a new paradigm. PLoS Biology. 2004, 2 (5): e133. PMC 406394
 . PMID 15138502. doi:10.1371/journal.pbio.0020133.
. PMID 15138502. doi:10.1371/journal.pbio.0020133.
- ^ Vazquez F; Vaucheret H; Rajagopalan R; Lepers C; Gasciolli V; Mallory AC; Hilbert J; Bartel DP; Crété P. Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Molecular Cell. 2004, 16 (1): 69–79. PMID 15469823. doi:10.1016/j.molcel.2004.09.028.
- ^ Watanabe T, Totoki Y, Toyoda A, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. May 2008, 453 (7194): 539–43. Bibcode:2008Natur.453..539W. PMID 18404146. doi:10.1038/nature06908.
- ^ Sontheimer EJ; Carthew RW. Silence from within: endogenous siRNAs and miRNAs. Cell. July 2005, 122 (1): 9–12. PMID 16009127. doi:10.1016/j.cell.2005.06.030.
- ^ Doran G. RNAi – Is one suffix sufficient?. Journal of RNAi and Gene Silencing. 2007, 3 (1): 217–19.
- ^ Pushparaj PN; Aarthi JJ; Kumar SD; Manikandan J. RNAi and RNAa — The Yin and Yang of RNAome. Bioinformation. 2008, 2 (6): 235–7. PMC 2258431
 . PMID 18317570. doi:10.6026/97320630002235.
. PMID 18317570. doi:10.6026/97320630002235.
- ^ Horwich MD; Li C; Matranga C; Vagin V; Farley G; Wang P; Zamore PD. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Current Biology. 2007, 17 (14): 1265–72. PMID 17604629. doi:10.1016/j.cub.2007.06.030.
- ^ Girard A; Sachidanandam R; Hannon GJ; Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006, 442 (7099): 199–202. Bibcode:2006Natur.442..199G. PMID 16751776. doi:10.1038/nature04917.
- ^ Horvath P; Barrangou R. CRISPR/Cas, the Immune System of Bacteria and Archaea. Science. 2010, 327 (5962): 167–70. Bibcode:2010Sci...327..167H. PMID 20056882. doi:10.1126/science.1179555.
- ^ Wagner EG; Altuvia S; Romby P. Antisense RNAs in bacteria and their genetic elements. Adv Genet. Advances in Genetics. 2002, 46: 361–98. ISBN 9780120176465. PMID 11931231. doi:10.1016/S0065-2660(02)46013-0.
- ^ Gilbert SF. Developmental Biology 7th. Sinauer. 2003: 101–3. ISBN 0-87893-258-5. OCLC 154656422.
- ^ Amaral PP; Mattick JS. Noncoding RNA in development. Mammalian genome : official journal of the International Mammalian Genome Society. October 2008, 19 (7–8): 454–92. PMID 18839252. doi:10.1007/s00335-008-9136-7.
- ^ Heard E; Mongelard F; Arnaud D; Chureau C; Vourc'h C; Avner P. Human XIST yeast artificial chromosome transgenes show partial X inactivation center function in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1999, 96 (12): 6841–46. Bibcode:1999PNAS...96.6841H. PMC 22003
 . PMID 10359800. doi:10.1073/pnas.96.12.6841.
. PMID 10359800. doi:10.1073/pnas.96.12.6841.
- ^ Batey RT. Structures of regulatory elements in mRNAs. Curr. Opin. Struct. Biol. 2006, 16 (3): 299–306. PMID 16707260. doi:10.1016/j.sbi.2006.05.001.
- ^ Scotto L; Assoian RK. A GC-rich domain with bifunctional effects on mRNA and protein levels: implications for control of transforming growth factor beta 1 expression. Mol. Cell. Biol. June 1993, 13 (6): 3588–97. PMC 359828
 . PMID 8497272.
. PMID 8497272.
- ^ Steitz TA; Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. U.S.A. 1993, 90 (14): 6498–502. Bibcode:1993PNAS...90.6498S. PMC 46959
 . PMID 8341661. doi:10.1073/pnas.90.14.6498.
. PMID 8341661. doi:10.1073/pnas.90.14.6498.
- ^ Xie J; Zhang M; Zhou T; Hua X; Tang L; Wu W. Sno/scaRNAbase: a curated database for small nucleolar RNAs and cajal body-specific RNAs. Nucleic Acids Res. 2007, 35 (Database issue): D183–7. PMC 1669756
 . PMID 17099227. doi:10.1093/nar/gkl873.
. PMID 17099227. doi:10.1093/nar/gkl873.
- ^ Omer AD; Ziesche S; Decatur WA; Fournier MJ; Dennis PP. RNA-modifying machines in archaea. Molecular Microbiology. 2003, 48 (3): 617–29. PMID 12694609. doi:10.1046/j.1365-2958.2003.03483.x.
- ^ Cavaillé J; Nicoloso M; Bachellerie JP. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature. 1996, 383 (6602): 732–5. Bibcode:1996Natur.383..732C. PMID 8878486. doi:10.1038/383732a0.
- ^ Kiss-László Z; Henry Y; Bachellerie JP; Caizergues-Ferrer M; Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996, 85 (7): 1077–88. PMID 8674114. doi:10.1016/S0092-8674(00)81308-2.
- ^ Daròs JA; Elena SF; Flores R. Viroids: an Ariadne's thread into the RNA labyrinth. EMBO Rep. 2006, 7 (6): 593–8. PMC 1479586
 . PMID 16741503. doi:10.1038/sj.embor.7400706.
. PMID 16741503. doi:10.1038/sj.embor.7400706.
- ^ Kalendar R; Vicient CM; Peleg O; Anamthawat-Jonsson K; Bolshoy A; Schulman AH. Large retrotransposon derivatives: abundant, conserved but nonautonomous retroelements of barley and related genomes. Genetics. 2004, 166 (3): 1437–50. PMC 1470764
 . PMID 15082561. doi:10.1534/genetics.166.3.1437.
. PMID 15082561. doi:10.1534/genetics.166.3.1437.
- ^ Podlevsky JD; Bley CJ; Omana RV; Qi X; Chen JJ. The telomerase database. Nucleic Acids Res. 2008, 36 (Database issue): D339–43. PMC 2238860
 . PMID 18073191. doi:10.1093/nar/gkm700.
. PMID 18073191. doi:10.1093/nar/gkm700.
- ^ Blevins, T.; Rajeswaran, R.; Shivaprasad, PV.; Beknazariants, D.; Si-Ammour, A.; Park, HS.; Vazquez, F.; Robertson, D.; Meins, F. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 2006, 34 (21): 6233–46. PMC 1669714
 . PMID 17090584. doi:10.1093/nar/gkl886.
. PMID 17090584. doi:10.1093/nar/gkl886.
- ^ Jana S; Chakraborty C; Nandi S; Deb JK. RNA interference: potential therapeutic targets. Appl. Microbiol. Biotechnol. 2004, 65 (6): 649–57. PMID 15372214. doi:10.1007/s00253-004-1732-1.
- ^ Schultz U; Kaspers B; Staeheli P. The interferon system of non-mammalian vertebrates. Dev. Comp. Immunol. 2004, 28 (5): 499–508. PMID 15062646. doi:10.1016/j.dci.2003.09.009.
- ^ Whitehead, K. A.; Dahlman, J. E.; Langer, R. S.; Anderson, D. G. Silencing or Stimulation? SiRNA Delivery and the Immune System. Annual Review of Chemical and Biomolecular Engineering. 2011, 2: 77–96. PMID 22432611. doi:10.1146/annurev-chembioeng-061010-114133.
- ^ Dahm R. Friedrich Miescher and the discovery of DNA. Developmental Biology. 2005, 278 (2): 274–88. PMID 15680349. doi:10.1016/j.ydbio.2004.11.028.
- ^ Caspersson T; Schultz J. Pentose nucleotides in the cytoplasm of growing tissues. Nature. 1939, 143 (3623): 602–3. Bibcode:1939Natur.143..602C. doi:10.1038/143602c0.
- ^ Ochoa S. Enzymatic synthesis of ribonucleic acid (PDF). Nobel Lecture. 1959.
- ^ Rich A; Davies, D. A New Two-Stranded Helical Structure: Polyadenylic Acid and Polyuridylic Acid. Journal of the American Chemical Society. 1956, 78 (14): 3548–3549. doi:10.1021/ja01595a086.
- ^ Holley RW, et al. Structure of a ribonucleic acid. Science. 1965, 147 (3664): 1462–65. Bibcode:1965Sci...147.1462H. PMID 14263761. doi:10.1126/science.147.3664.1462.
- ^ Siebert S. Common sequence structure properties and stable regions in RNA secondary structures (PDF). Dissertation, Albert-Ludwigs-Universität, Freiburg im Breisgau: 1. 2006. (原始内容 (PDF)存档于March 9, 2012).
- ^ Szathmáry E. The origin of the genetic code: amino acids as cofactors in an RNA world. Trends Genet. 1999, 15 (6): 223–9. PMID 10354582. doi:10.1016/S0168-9525(99)01730-8.
- ^ Fiers W, et al. Complete nucleotide-sequence of bacteriophage MS2-RNA: primary and secondary structure of replicase gene. Nature. 1976, 260 (5551): 500–7. Bibcode:1976Natur.260..500F. PMID 1264203. doi:10.1038/260500a0.
- ^ Napoli C; Lemieux C; Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990, 2 (4): 279–89. PMC 159885
 . PMID 12354959. doi:10.1105/tpc.2.4.279.
. PMID 12354959. doi:10.1105/tpc.2.4.279.
- ^ Dafny-Yelin M; Chung SM; Frankman EL; Tzfira T. pSAT RNA interference vectors: a modular series for multiple gene down-regulation in plants. Plant Physiol. December 2007, 145 (4): 1272–81. PMC 2151715
 . PMID 17766396. doi:10.1104/pp.107.106062.
. PMID 17766396. doi:10.1104/pp.107.106062.
- ^ Ruvkun G. Glimpses of a tiny RNA world. Science. 2001, 294 (5543): 797–99. PMID 11679654. doi:10.1126/science.1066315.
- ^ Fichou Y; Férec C. The potential of oligonucleotides for therapeutic applications. Trends in Biotechnology. 2006, 24 (12): 563–70. PMID 17045686. doi:10.1016/j.tibtech.2006.10.003.
External links
[编辑]- RNA World website Link collection (structures, sequences, tools, journals)
- Nucleic Acid Database Images of DNA, RNA and complexes.
- RNA Calculators
